On the morning of March 8, local time, in Barcelona, Spain, RemeGen gave an oral presentation on clinical study of Disitamab Vedotin for the treatment of Cervical Cancer at the 25th European Congress on Gynecological Oncology (ESGO 2024 Congress), which was the first time for Chinese original ADC to be presented in an important international gynecological oncology congress.

This national multicenter, open-label phase 2 study was led by Professor Wu Lingying, Cancer Hospital, Chinese Academy of Medical Sciences, and this time Professor Miao Jingwei from Beijing Obstetrics and Gynecology Hospital, Capital Medical University gave an in-depth presentation on behalf of the research team at the congress. The preliminary study results were discussed by the participating experts, and further results are expected to come out to open a window of life for the treatment of recurrent/metastatic cervical cancer.
ESGO is the largest academic congress on gynecological oncology in Europe, attracting thousands of experts and scholars from all over the world in the field of gynecological cancer treatment, research, etc. every year. At the oral presentation session, the latest high-quality research data will be released, and global experts will discuss and share views.

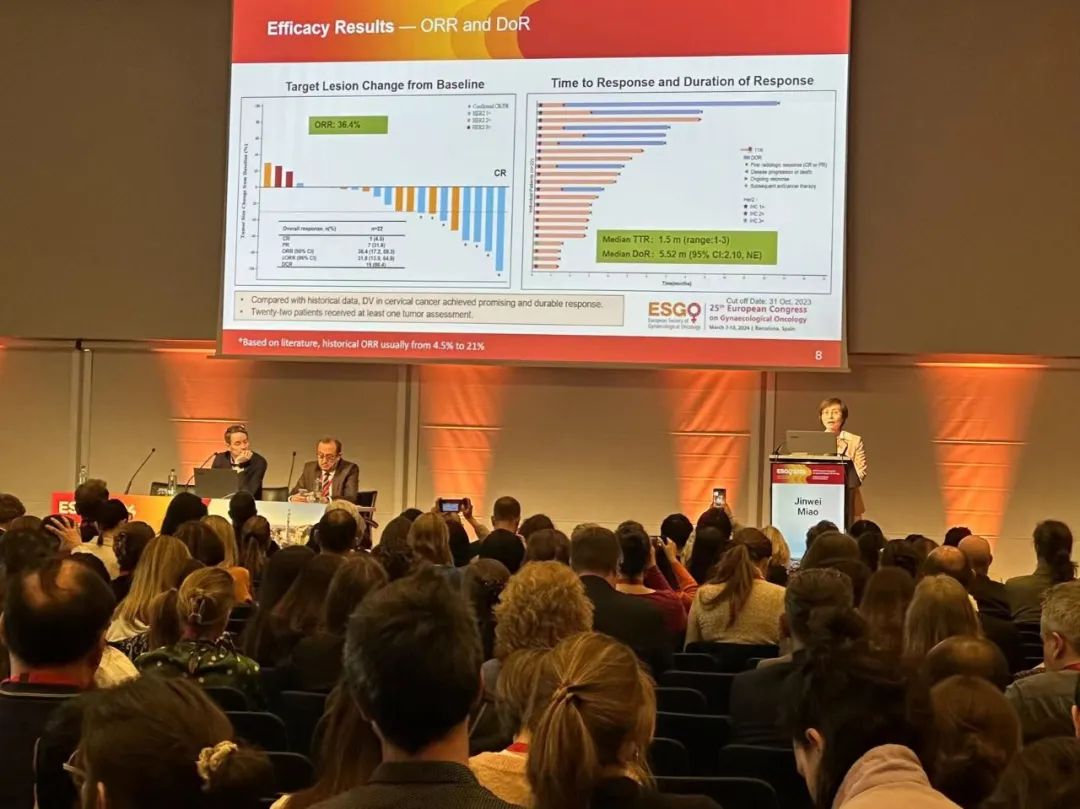
This oral presentation was the results of an open-label, multicenter Phase 2 basket design study of Disitamab Vedotin to evaluate the efficacy and safety of Disitamab Vedotin monotherapy in the treatment of HER2-expressing gynecologic malignancies. The cervical cancer cohort includes patients with recurrent or metastatic cervical cancer who have progressed on at least 1L anti-tumor therapy and have HER2 IHC ≥ 1+. The primary endpoint is objective response rate (ORR) by Independent Review Committee (IRC), with secondary endpoints including ORR by investigator, duration of response (DoR), disease control rate (DCR), progression free survival (PFS), overall survival (OS), and safety.
As of October 31, 2023, 25 patients with cervical cancer were enrolled with a median age of 56 years. Most patients had a baseline ECOG performance score of 1. 18 patients had a primary FIGO stage (a widely used staging criterion for gynecologic tumors) of IIB or higher. 16 patients had squamous cell carcinoma, and 9 had adenocarcinoma. More than half (52%) of the patients had received 2 or more lines of therapy. Among the 22 efficacy evaluable patients, the ORR was 36.4%, the DCR was 86.4%, the mDoR was 5.52 months, the mPFS was 4.37 months, the mOS was immature, and the 1-year OS rate was 66%. Disitamab Vedotin demonstrated manageable safety and positive efficacy in patients with HER2-expressing recurrent or metastatic cervical cancer, suggesting that it to be a promising new treatment for such patients.
Cervical cancer is a common gynecological malignancy. According to the data published by the International Agency for Research on Cancer (IARC), there were 111,820 new cases of cervical cancer and 61,579 deaths in 2022, with both incidence and mortality increasing. Currently, for patients with advanced, recurrent metastases, limited treatment patterns and high recurrence rates often lead to poor prognosis and an urgent need to improve patient survival. As a new generation of anti-HER2 ADC, Disitamab Vedotin has been approved for the treatment of gastric cancer and urothelial cancer in China, and has achieved encouraging results in a variety of malignant tumor studies. The data at this ESGO congress also indicate that it has good anti-tumor activity and sustained remission in the field of gynecological malignancies such as cervical cancer, and is expected to bring a new breakthrough in the later-line treatment of advanced cervical cancer.
Dr. Jianmin Fang, CEO of RemeGen, commented, “As the first original ADC in China, the study results of Disitamab Vedotin have been presented at major domestic and international conferences for many times. I am delighted to share an oral presentation on our latest study results in the treatment of cervical cancer at ESGO 2024. RemeGen will advance the next research and exploration in order to bring more benefits to patients in urgent need of help as soon as possible and lead to a new era of HER2-ADC treatment for gynecological cancers.”
