On October 15, 2024, RemeGen Co., Ltd. (“RemeGen”) announced that the Center for Drug Evaluation (CDE) of China’s National Medical Products Administration (NMPA) had officially accepted and granted priority review for its Biologics License Application (BLA) of Disitamab Vedotin for a new indication: HER2-positive (IHC result of 3+ or FISH+) advanced breast cancer with liver metastasis previously treated with Trastuzumab or its biosimilar or taxanes. Disitamab Vedotin is a novel antibody-drug conjugate (ADC) initially developed by RemeGen.
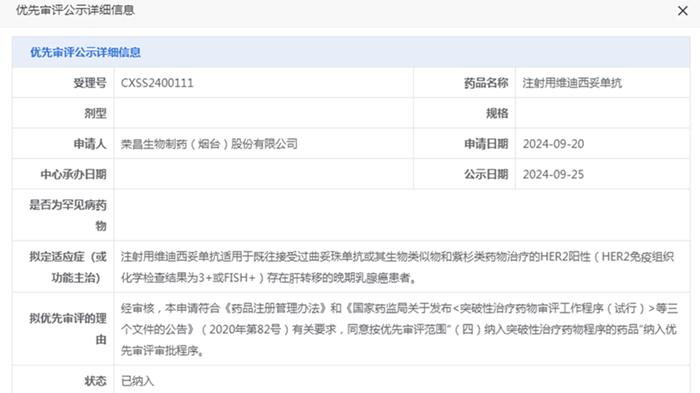
Disitamab Vedotin was granted breakthrough therapy designation for this same indication by CDE in June, 2021. Priority review and breakthrough therapy designations are two important pathways regulatory authorities employ to expedite drug review process. The priority review designation is designed to accelerate the review of new drugs with potential to significantly improve the treatment of diseases that pose serious threats to patients’ lives or substantially diminish life quality. The breakthrough therapy designation means shorter review time for new drugs demonstrating markedly superior efficacy in clinical trials, thereby addressing unmet clinical needs. These designations are typically granted to investigational agents that exhibit favorable efficacy and safety profiles in clinical trial and may offer substantial improvements in clinical practice.
The present BLA is based on the data from an open-label, controlled, parallel, multicenter Phase Ⅲ clinical trial conducted in China (RC48-C006, NCT03500380) to evaluate efficacy and safety of Disitamab Vedotin for Injection versus Lapatinib plus Capecitabine in the treatment of patients with HER2-positive advanced breast cancer. The results demonstrated that Disitamab Vedotin can significantly improve progression-free survival (PFS) compared with Lapatinib plus Capecitabine. More details about this study will be released at San Antonio Breast Cancer Symposium (SABCS) in December, 2024.
Breast cancer is the second most common cancer type globally. According to a global cancer statistics report, an estimated 2.31 million new cases of breast cancer were diagnosed in 2022, ranking second in the incidence list. In China, numbers of new cases and deaths in 2022 were 357,000 and 75,000 respectively, indicating a significant disease burden. Despite the availability of the diversified therapeutic options now, managing breast cancer remains a challenge due to factors like drug tolerance, adverse events and the absence of specific therapies. The situation is particular concerning for patients with advanced breast cancer that has spread to liver, given the limited treatment options and poor prognosis. The acceptance of BLA for Disitamab Vedotin may bring new hopes to this community. In addition to the HER2-positive patients, RemeGen is pressing forward another Phase Ⅲ clinical trial (NCT04400695) on Disitamab Vedotin among individuals with HER2-low breast cancer.
To date, Disitamab Vedotin have been approved by NMPA and added to China’s National Reimbursement Drug List for two indications, i.e. HER2-overexpressing locally advanced or metastatic gastric carcinoma previously treated with at least two lines of systemic chemotherapies and HER2-overexpressing locally advanced or metastatic urothelium carcinoma previously treated with platinum-based chemotherapies. Furthermore, RemeGen is exploring the potential of Disitamab Vedotin as front-line therapies for gastric carcinoma and urothelium carcinoma, and relevant Phase Ⅲ clinical trials are currently ongoing.
