On 15th September, 2024, the latest data of two significant studies on Disitamab Vedotin was released in form of mini oral presentation and poster at 2024 ESMO Congress in Barcelona, Spain, demonstrating the promising efficacy and manageable safety profiles of “Disitamab Vedotin + PD-1 inhibitor” as first-line treatment in patients with advanced urothelial carcinoma.
Median OS of 33.1 Months,
20 Months Longer Than
First-line Platinum-containing Chemotherapy
The study presented by poster was a phase Ib/II clinical trial of Disitamab Vedotin plus Toripalimab (T) in treatment of HER2-expressing unresectable locally advanced or metastatic urothelial carcinoma (la/mUC) (C014) led by Professor Jun Guo from Beijing Cancer Hospital. The median overall survival (OS) of this study was 33.1 months, 20 months longer than that (13 months) of the first-line platinum-containing chemotherapy. This data, disclosed for the first time, suggests the combination therapy of “Disitamab Vedotin + PD-1 inhibitor” could improve survival of urothelial carcinoma patients.
As of 1st March, 2024, this study has enrolled 41 subjects with a median age of 66. 41.5% of the enrolled subjects suffered from lung metastases and 24.2% liver metastases. As the data demonstrated, the confirmed objective response rate (cORR) in all subjects was 75.0% and that in subjects with HER2 IHC 2+ was 88.9%. The median follow-up period was 33.2 months, the median OS 33.1 months and the median progression-free survival (mPFS) 9.3 months.
OS is a gold-standard measure of the clinical benefit in survival. The long-term follow-up of the above study demonstrated that patients on the “Disitamab Vedotin-Toripalimab” combination therapy had superior tumor response and got a remarkable clinical benefit of longer-than-ever OS. This encouraging finding indicated that this combination therapy could improve survival of patients and therefore had a great potential to be a favorable first-line treatment option for advanced urothelial carcinoma. The enrollment of the randomized controlled phase 3 study of Disitamab Vedotin with PD-1 inhibitor (Toripalimab) vs platinum-containing chemotherapy as first-line treatment in patients with advanced urothelial carcinoma has been completed and the data is expected to read out in the first half of 2025.
cORR up to 78.6%
First Presentation of Favorable Efficacy of Disitamab Vedotin
in Global Patients
In August 2021, Seagen Inc., a world-renowned biotechnology company, and RemeGen Co., Ltd. (“RemeGen”) entered into an exclusive worldwide (excluding Asia-Pacific region with Japan and Singapore excepted) licensing agreement on Disitamab Vedotin. Pursuit to the agreement, RemeGen will receive up to US$2.6 billion in upfront and milestone payments and a tiered royalty at percentages ranging from high single-digit to mid-teens on future sales by Seagen. After Pfizer’s acquisition of Seagen, RemeGen and Pfizer are working together to proceed the global multicenter clinical trials.
At 2024 ESMO Congress, Dr. M. Galsky from Mount Sinai Medical Center, New York, US gave a mini oral presentation about RC48G001 Cohort C, the global multicenter clinical study of Disitamab Vedotin combined with Pembrolizumab as first-line treatment of HER2-expressing la/uMC, presenting the encouraging efficacy of “Disitamab Vedotin + PD-1 inhibitor” in global patients for the first time, especially in HER2-low patients.
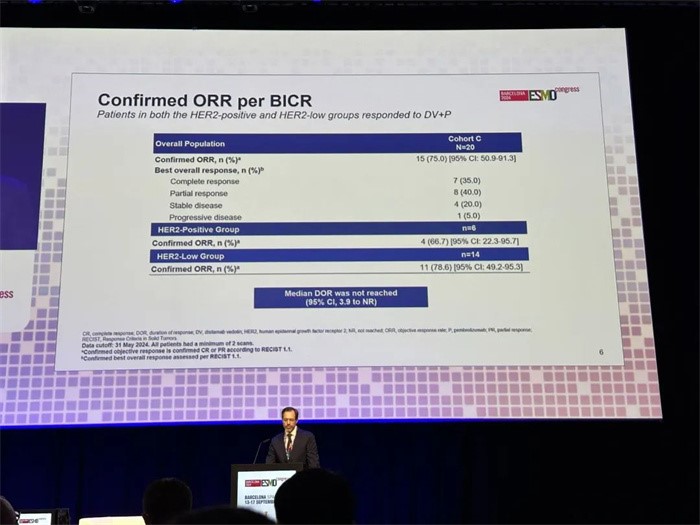
This is a single-arm, multi-cohort and open-label global multicenter phase 2 clinical study. The first cohort enrolled 20 subjects with the median age of 75, among whom 30% were HER2-positive and 70% HER2-low. The cORR reached 75% in this cohort as a whole and up to 78.6% among the HER2-low subjects. Median duration of response (DOR) has not reached yet. Disitamab Vedotin combined with Pembrolizumab showed manageable safety profile.
This global multicenter clinical study is still enrolling subjects. In addition, the global multicenter phase 3 study (DV-001) of Disitamab Vedotin combined with Pembrolizumab vs chemotherapy in previously untreated HER2-expressing patients with locally advanced or metastatic urothelial carcinoma that expresses HER2 is proceeding smoothly.
“ADC+PD-1 Inhibitor”
Reshaping the Treatment Landscape of Urothelial Carcinoma
Urothelial carcinoma accounts for over 90% of all cases of bladder cancer and is the second most common malignancy of urinary system following kidney cancer. According to the latest data published by International Agency for Research on Cancer (IARC) of World Health Organization (WTO) in April 2024, the whole world saw 614,000 new cases and 220,000 deaths of bladder cancer in 2022. For patients with unresectable locally advanced urothelial carcinoma, platinum-containing chemotherapy remains the preferred first-line treatment. But the median OS in these patients is only about 1 year, around half of the patients are cisplatin-resistant, and the prognosis is poor. It is indicated that patients with locally advanced or metastatic urothelial carcinoma are in urgent need of alternative treatment options with better efficacy. Now experts around the world are exploring new regimens of antibody drug conjugate (ADC) with immunotherapy as first-line treatment in patients with advanced urothelial carcinoma.
HER2 plays an important role in the pathogenesis and development of urothelial carcinoma. 86% of the patients are confirmed with HER2 expression (HER2 IHC 1+ and higher). [1] As a new HER2-targeted ADC, Disitamab Vedotin shows promising potential for treating patients with HER2-overexpressing locally advanced or metastatic urothelial carcinoma. It comprises a HER2-targeted monoclonal antibody conjugated to a highly efficacious cytotoxic molecule of MMAE, allowing it to kill HER2-expressing tumor cells selectively. Additionally, it can kill HER2-low tumor cells through bystander killing effect. Besides, ADC has the capacity to activate the immune response through immunogenic cell death, enabling synergistic effect of the combination of Disitamab Vedotin and immunotherapy.
At this meeting, a total of 11 studies of Disitamab Vedotin were unveiled, showcasing its breakthrough progress in treatment of multiple cancers. There will be more detailed interpretation and related updates on other selected studies. Please stay tuned for further information.
