News
-
03 2025.06
2025 ASCO | Selected in Rapid Oral Abstract Presentation: Disitamab Vedotin as First-Line Therapy for HER2-Expressing Locally Advanced or Metastatic Gastric Cancer Achieves Efficacy Breakthrough
-
12 2025.05
Poised to Reshape Treatment Landscape: A Phase 3 Clinical Trial on Disitamab Vedotin as a First-Line Therapy for HER2-Expressing Locally Advanced or Metastatic Urothelial Carcinoma Reached its Primary Endpoints of PFS and OS
-
09 2025.05
RemeGen’s Disitamab Vedotin Gains Approval in China for its Third Indication Making it the First ADC Approved for HER2-Positive Advanced Breast Cancer with Liver Metastasis Globally
-
14 2025.02
ASCO GU|RemeGen Announced Highly Encouraging Data from the Phase II Clinical Trial Evaluating Disitamab Vedotin plus Immunotherapy as Perioperative Regimen for Bladder Cancer
-
07 2025.01
Published in Annals of Oncology: Disitamab Vedotin Combined with PD-1 Inhibitor is a Promising Treatment Option for Locally Advanced or Metastatic Urothelial Carcinoma
Mechanism of Action
ADCs are a type of cancer treatment designed to specifically and directly deliver chemotherapies to tumor cells while sparing healthy cells. The concept of ADCs is based on exploiting the high specificity of a monoclonal antibody toward a selected tumor cell-surface antigen and enhancing the cell-killing capacity of the antibody by attaching a highly cytotoxic agent. Typically, several molecules of a highly potent cytotoxic compound are linked to each antibody molecule to enhance its activity, while retaining the favorable pharmacokinetic and pharmacodynamic properties of the antibody. The key to this type of therapy is getting three distinct molecules—antibody, active drug and linker—to work together.
Unlike traditional chemotherapy that indiscriminately damages healthy cells as well as tumor cells, ADC utilizes monoclonal antibody to bind to tumor-specific antigen targets and then delivers the chemotherapy, a highly potent cytotoxic agent, to kill tumor cells. In this way, ADCs may significantly benefit cancer patients by causing less adverse effects (AEs) or severe adverse effects (SAEs).
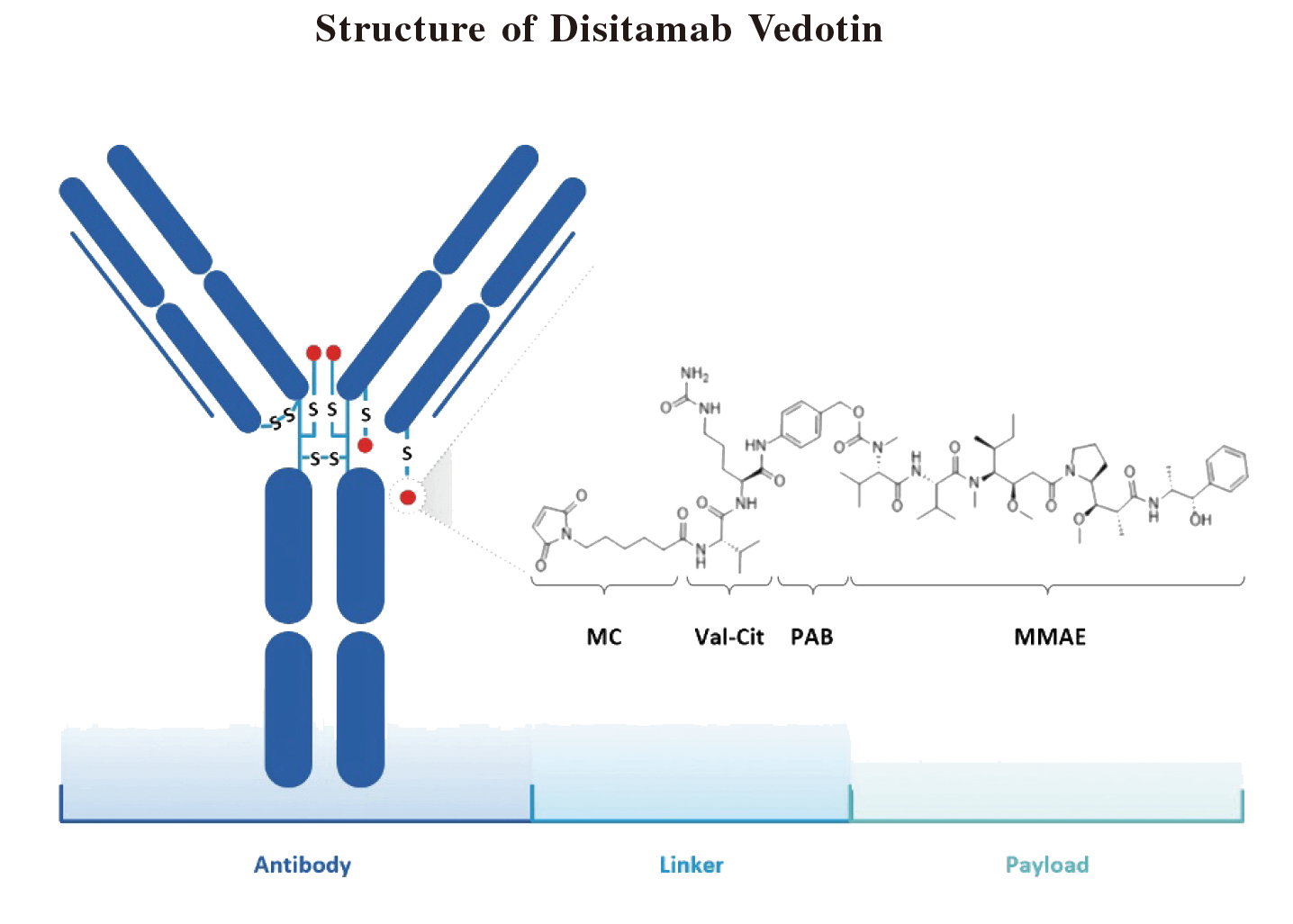
Abbreviation: MC = maleimidocaproyl; MMAE = monomethyl auristatin E; PAB = p-aminobenzyl. Note: MC-Val-Cit-PAB is a cathepsin cleavable ADC linker.
As illustrated in the diagram above, in Disitamab Vedotin, a novel humanized HER2 antibody and monomethyl auristatin E (MMAE), a potent tubulin binder with a half maximal inhibitory concentration (IC50) in the subnanomolar range, as the cytotoxic payload, are conjugated to each other through a cathepsin cleavable linker, with optimized drug-antibody ratio. The anti-HER2 antibody allows Disitamab Vedotin to selectively deliver the anti-cancer agent MMAE to HER2-expressing tumor cells.
HER2 is a member of the epidermal growth factor receptor (EGFR) family. It is expressed in many tissues, including the breast, gastrointestinal tract, kidney and heart. Its major role in these tissues is to promote cell proliferation and suppress apoptosis. Amplification of the HER2 gene and overexpression of its product may drive excessive or uncontrolled cell growth and tumorigenesis. Our clinical data support the scientific view that the HER2 pathway may play a key role in the treatment of many cancer types with tumors that express HER2 antigen, such as breast, gastric, lung and urothelial cancers.
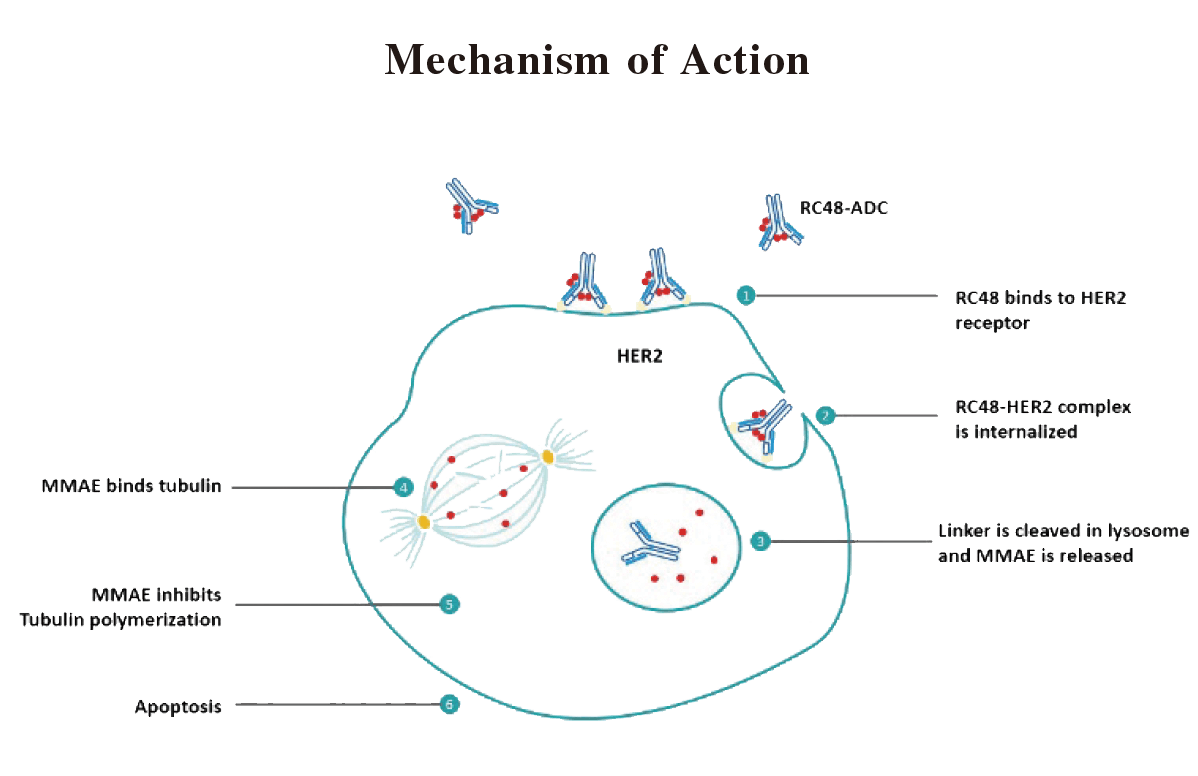
Disitamab Vedotin comprises a novel HER2 monoclonal antibody that targets a different epitope of, and also shows a high binding affinity for, HER2 receptors on the tumor cell. Once Disitamab Vedotin binds to its target (HER2) which is expressed on tumor cell surface, through its antibody component (disitamab), the ADC-HER2 complex is internalized by the tumor cell via endocytosis. The linker connecting antibody and cytotoxic payload is then cleaved in the presence of lysosomal protease. Once the payload, MMAE, is released into cytosol, it binds tubulin and inhibits its polymerization, which triggers apoptosis or the programmed cell death of the HER2-expressing tumor cell. MMAE, once released, also has the capacity to kill neighboring tumor cells (whether HER2-expressing or not), which is known as the bystander-killing effect. Studies have found that ADCs with highly membrane-permeable payloads, such as MMAE, have a more potent bystander killing effect than those ADCs that have low membrane-permeable payloads, indicating a higher anti-tumor potential for our Disitamab Vedotin.
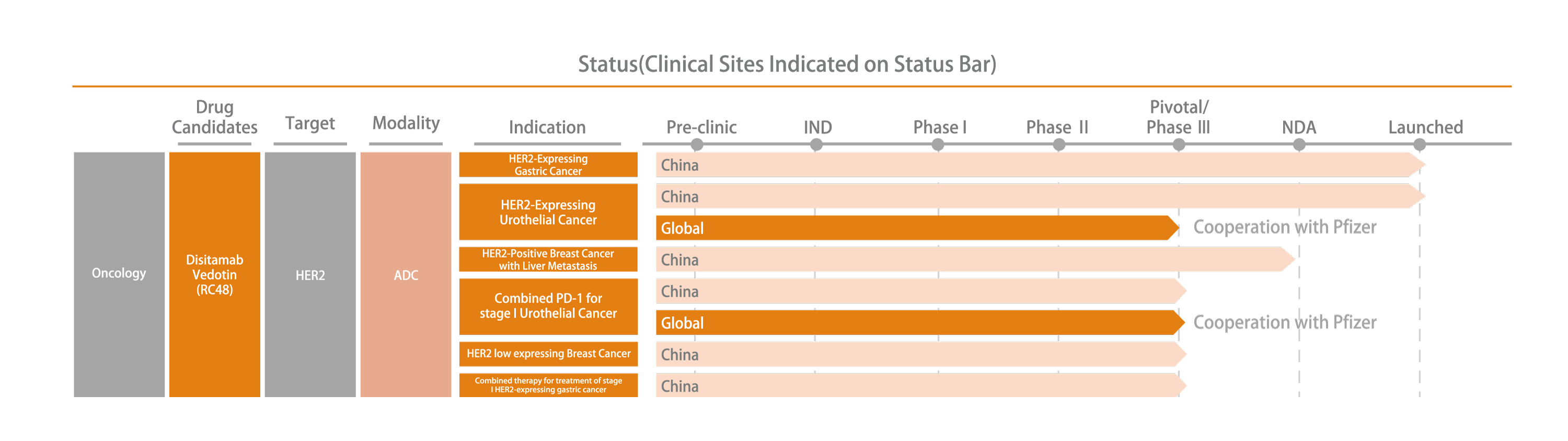
Indication
Disitamab Vedotin is the first domestically produced ADC drug approved for marketing in China. It is also the first ADC drug in China to be dually recognized as a breakthrough therapy by FDA, US and NMPA of China. Disitamab Vedotin’s indications for gastric cancer and urothelial cancer were approved for marketing by NMPA in June 2021 and December 2021 respectively, and entered the National Reimbursement Drug List in January 2023.
Competitive Advantages of Disitamab Vedotin
• Structural design advantages
- Using independently developed and optimized monoclonal antibodies with higher target affinity
- Improved overall killing effect of tumor tissue based on " by-standard effect " and improved blood stability and safety of ADC drugs by using optimized ligands that can be digested by enzymes
• Production advantages
The development and production of ADC drugs involve a series of key technologies such as antibody macromolecule drugs, efficient chemical drugs, methods of conjugate and so on, which are of great technical difficulty and high technological requirements. Through independent research and development, we have now broken through the technical bottleneck of Disitamab Vedotin in the aspects of antibody preparation, binding of linker and cytotoxin, process of conjugate and preparation optimization, pharmacological research, quality evaluation and large-scale production, and we have accumulated all the key technologies of ADC drug from the research and development source to commercial production. Our current production capacity of ADC drugs can fully satisfied the needs of clinical experimental drugs and the current industrialization, forming a high technical barrier.
• Preclinical and clinical efficacy advantages
Based on the optimized target affinity and the " by-standard effect" on tumor tissue, Disitamab Vedotin selectively inhibits the proliferation of HER2-positive tumor cells, induces cell cycle arrest and apoptosis, and has demonstrated good efficacy and safety in preclinical and clinical trials.