About Us
RemeGen is committed to the discovery, development, production and commercialization of first-in-class and best-in-class biological drugs, and has developed a series of innovative biologics with significant clinical value for major diseases such as autoimmunity, oncology, and ophthalmology.
RemeGen Co., Ltd. (Yantai) was co-founded in 2008 by Mr. Wang Weidong, founder of Rongchang Pharmaceuticals, and Dr. Jianmin Fang a Canadian-American scientist. RemeGen is headquartered in Yantai, Shandong Province, China, with R&D centers and offices in China and the United States.
Learn More
-
2A+H Dual-listed

-
8Clinical Pipelines

-
40 +Clinical Trials

-
600 +Patent Applications

Product Pipeline
Learn More
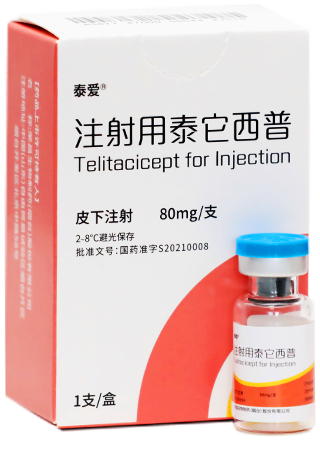
Telitacicept
The World’s First Innovative Dual-target Biological Drug to Treat Systemic Lupus Erythematosus The World’s First Innovative Dual-target Biological Drug to Treat Systemic Lupus Erythematosus Trade name: Tai’ai ® Synonym: RC18 Target: BLyS and APRIL Learn More
Disitamab Vedotin
China’s First Independently Developed Innovative ADC China’s First Independently Developed Innovative ADC Trade name: Aidixi® Synonym: RC48 Target: HER2 Learn More
Top News Express
Learn More
-

ESMO Congress 2025 Presidential Symposium Oral Presentation | Disitamab Vedotin Achieves Major Breakthrough as First-Line Treatment for Urothelial Carcinoma
2025-10-20
BERLIN, Oct. 20, 2025 /PRNewswire/ -- At the 2025 European Society for Medical Oncology (ESMO) Congress, a Phase III clinical study on disitamab vedotin plus toripalimab versus chemotherapy as first-line treatment for HER2-expressing locally adv
-

Results of China Phase III Clinical Study of Telitacicept for Generalized Myasthenia Gravis Selected for Oral Presentation at 2025 AANEM Annual Meeting
2025-09-17
YANTAI, China, Sept. 17, 2025 /PRNewswire/ -- RemeGen (688331.SH/09995.HK) announced that the 48-week open-label extension (OLE) data from China Phase III clinical study of telitacicept (RC18, brand name: 泰爱®, a BLyS/APRIL dual-targ
-

RemeGen and Santen Enter into Exclusive Licensing Agreement for Ophthalmic Innovative Drug RC28-E in Greater China and Asian countries
2025-08-18
YANTAI, China, Aug. 18, 2025 /PRNewswire/ -- RemeGen Co., Ltd. (Stock Code: 688331.SH/09995.HK, "RemeGen"), a leading Chinese biopharmaceutical company, announced today that it has entered into an agreement with Santen Pharmaceuti
-

Telitacicept Meets Primary Endpoint in Phase III Trial for Primary Sjögren's Syndrome in China
2025-08-13
YANTAI, China, Aug. 13, 2025 /PRNewswire/ -- On August 13th, Remegen (688331.SH/09995.HK) announced that its global first-in-class BLyS (BAFF)/APRIL dual-target fusion protein drug, Telitacicept, met the primary endpoint in its Phase I
-

RemeGen's Independently-Developed Bispecific Antibody RC148 Approved to Proceed Phase II Clinical Trial in US by FDA
2025-08-08
YANTAI, China, Aug. 8, 2025 /PRNewswire/ -- On August 8th, RemeGen Co., Ltd. (688331.SH/09995.HK) announces clearance of IND application by Food and Drug Administration (FDA) for phase II clinical trials for its independently-developed
-
2025 ASCO | Selected in Rapid Oral Abstract Presentation: Disitamab Vedotin as First-Line Therapy for HER2-Expressing Locally Advanced or Metastatic Gastric Cancer Achieves Efficacy Breakthrough
2025-06-03
Chicago, US, June 2, 2025 (UTC-8)—In a rapid oral abstract session of the 2025 ASCO Annual Meeting, Dr. Lin Shen from Beijing Cancer Hospital presented the result of a Phase 2 clinical trial conducted in China to evaluate the efficacy and safety of
-

2025-10-20
ESMO Congress 2025 Presidential Symposium Oral Presentation | Disitamab Vedotin Achieves Major Breakthrough as First-Line Treatment for Urothelial Carcinoma/p>
-
2025-09-17
Results of China Phase III Clinical Study of Telitacicept for Generalized Myasthenia Gravis Selected for Oral Presentation at 2025 AANEM Annual Meeting/p>
-
2025-08-18
RemeGen and Santen Enter into Exclusive Licensing Agreement for Ophthalmic Innovative Drug RC28-E in Greater China and Asian countries/p>
Investor Services
Learn More
Financial Reports

Corporate Governance

Circulars

STAR Market stock code
荣昌生物:688331.SH
HKEX stock code
荣昌生物:09995.HK
Embrace Outstanding Talents
Learn More
Embracing a “people first” talent concept, we have established a mature employee career development system, providing diverse learning and training programs, offering competitive compensation and benefits, and creating a broad platform for the development of various talents.